PHOTOFRIN is indicated for treatment of microinvasive endobronchial NSCLC in patients for whom surgery and radiotherapy are not indicated.
The efficacy of PHOTOFRIN® PDT was also evaluated in the treatment of microinvasive endobronchial tumors in 62 inoperable patients in three noncomparative studies.
As shown below, the complete tumor response rate, biopsy-proven at least 3 months after treatment, was 50%, median time to tumor recurrence was more than 2.7 years, median survival was 2.9 years and disease-specific survival was 4.1 years.
Overall Efficacy in Patients with Superficial Endobronchial Tumors
| EFFICACY PARAMETER | PDT n=11 | PDT n=62 |
|---|---|---|
| COMPLETE TUMOR RESPONSE, BIOPSY-PROVEN AT 3 MONTHS | ||
| Number of Patients (%) | 3 (27) | 31 (50)a |
| TIME TO TUMOR RECURRENCE IN PATIENTS WITH COMPLETE RESPONSE | ||
| Number of Patients (%) with Recurrences | 1 (33) | 11 (35) |
| Median Time to Tumor Recurrence | >2.7 years | |
| [95% Confidence Interval] | [1.6, b] | |
| SURVIVAL | ||
| Number of Patients (%) who Died of Any Cause | 4 (36) | 32 (52) |
| Median Survival | 2.9 years | |
| [95% Confidence Interval] | [2.1, 5.7] | |
| DISEASE-SPECIFIC SURVIVAL | ||
| Number of Patients (%) who Died of Lung Cancer | 3 (27) | 22 (35) |
| Median Disease-Specific Survival | 4.1 years | |
| [95% Confidence Interval] | [2.5,—]b |
aNot included are an additional 18 patients (6 patients not eligible for surgery or radiotherapy) who had complete tumor
responses which were documented earlier than 3 months after treatment.
bThe upper limit of the confidence interval could not be estimated due to an insufficient number of patients whose tumors
recurred (Time to Tumor Recurrence) or who died (Survival).
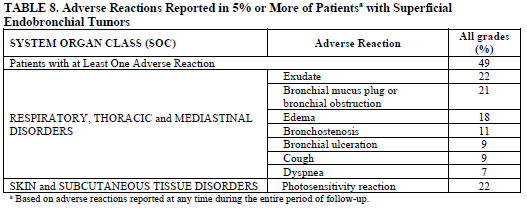
Adverse Reactions Reported in 5% or More of Patientsa with Superficial Endobronchial Tumors
| SYSTEM ORGAN CLASS (SOC) | Adverse Reaction | All grades (%) |
|---|---|---|
| Patients with at Least One Adverse Reaction | 49 | |
| RESPIRATORY, THORACIC and MEDIASTINAL DISORDERS | Exudate | 22 |
| Bronchial mucus plug or bronchial obstruction | 21 | |
| Edema | 18 | |
| Bronchostenosis | 11 | |
| Bronchial ulceration | 9 | |
| Cough | 9 | |
| Dyspnea | 7 | |
| SKIN and SUBCUTANEOUS TISSUE DISORDERS | Photosensitivity reaction | 22 |
aBased on adverse reactions reported at any time during the entire period of follow-up.
IMPORTANT SAFETY INFORMATION
Esophageal Cancer
PHOTOFRIN® is indicated for the palliation of patients with completely obstructing esophageal cancer, or of patients with partially obstructing esophageal cancer who, in the opinion of their healthcare provider, cannot be satisfactorily treated with Nd:YAG laser therapy.
Endobronchial Cancer
PHOTOFRIN is indicated for the treatment of microinvasive endobronchial non-small-cell lung cancer (NSCLC) in patients for whom surgery and radiotherapy are not indicated.
PHOTOFRIN is indicated for the reduction of obstruction and palliation of symptoms in patients with completely or partially obstructing endobronchial NSCLC.
High-Grade Dysplasia in Barrett’s Esophagus
PHOTOFRIN is indicated for the ablation of high-grade dysplasia in Barrett’s esophagus patients who do not undergo esophagectomy.
IMPORTANT WARNINGS AND PRECAUTIONS USING PHOTOFRIN® INCLUDE:
Gastroesophageal Fistula and Perforation: Do not initiate PHOTOFRIN with photodynamic therapy (PDT) in patients with esophageal tumors eroding into the trachea or bronchial tree or bronchial wall.
Pulmonary and Gastroesophageal Hemorrhage: Assess patients for tumors eroding into a pulmonary blood vessel and esophageal varices. Do not administer light directly to an area with esophageal varices.
High-Grade Dysplasia (HGD) in Barrett’s Esophagus (BE): After treatment of HGD in BE, conduct endoscopic biopsy surveillance every 3 months, until 4 consecutive negative evaluations for HGD have been recorded.
Photosensitivity and Ocular Photosensitivity: Observe precautions to avoid exposure of skin and eyes to direct sunlight or bright indoor light for at least 30 days. Instruct patients when outdoors to wear dark sunglasses which have an average light transmittance of <4% for at least 30 days and until ocular sensitivity resolves.
Use Before or After Radiotherapy: Allow 2-4 weeks between PDT and subsequent radiotherapy.
Chest Pain: Substernal chest pain can occur.
Airway Obstruction and Respiratory Distress: Administer with caution to patients with tumors in locations where treatment-induced inflammation can obstruct the main airway. Monitor patients closely between the laser light therapy and the mandatory debridement bronchoscopy for any evidence of respiratory distress.
Esophageal Strictures: Esophageal strictures can occur.
Hepatic and Renal Impairment: Patients with hepatic or renal impairment may need longer precautionary measures for photosensitivity.
Thromboembolism: Thromboembolic events can occur.
Embryo-Fetal Toxicity: May cause embryo-fetal toxicity. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception.
MOST COMMON ADVERSE REACTIONS reported during clinical trials (>10% of patients) are:
Esophageal Cancer: Anemia, pleural effusion, pyrexia, constipation, nausea, chest pain, pain, abdominal pain, dyspnea, photosensitivity reaction, pneumonia, vomiting, insomnia, back pain, pharyngitis.
Obstructing Endobronchial Cancer: Dyspnea, photosensitivity reaction, hemoptysis, pyrexia, cough, pneumonia.
Superficial Endobronchial Tumors: Exudate, photosensitivity reaction, bronchial obstruction, edema, bronchostenosis.
High-Grade Dysplasia in Barrett’s Esophagus: Photosensitivity reaction, esophageal stenosis, vomiting, chest pain, nausea, pyrexia, constipation, dysphagia, abdominal pain, pleural effusion, dehydration.
Other Photosensitizing Agents: May increase the risk of photosensitivity reaction.
Lactation: Because of the potential for serious adverse reactions in the breastfed infant, advise patients that breastfeeding is not recommended during treatment with PHOTOFRIN and for 5 months after the last dose.
Please see full Prescribing Information for PHOTOFRIN (porfimer sodium) for Injection at: www.photofrin.com.
FOR MORE INFORMATION about PHOTOFRIN, or if there are any questions regarding the information provided, visit www.photofrin.com or please contact the Medical Information Department at 1-866-248-2039. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
PHOTOFRIN® and OPTIGUIDE® are registered trademarks of Concordia Laboratories Inc.
Pinnacle Biologics™ and the logo of Pinnacle Biologics™ are trademarks of Pinnacle Biologics, Inc.
PHOTOFRIN® is distributed in the United States by Pinnacle Biologics, Inc., Bannockburn, IL 60015
All rights reserved.
Please see accompanying full Prescribing Information for Photofrin®