PDT IN DIFFERENT STAGES OF NON-SMALL CELL LUNG CANCER (NSCLC)
- It is one of the modalities for definitive therapy for carcinoma in situ and microinvasive (superficial) NSCLC2
- Symptomatic management Stage I or II3,4
- Can be used for induction for Stage IIIA or III4
- Palliation5
The combined results of Two randomized multicenter studies conducted to compare the safety and efficacy of PHOTOFRIN PDT versus Nd:YAG laser therapy for reduction of obstruction and palliation of symptomatic patients with partially or completely obstructing endobronchial non-small-cell lung cancer are shown below.
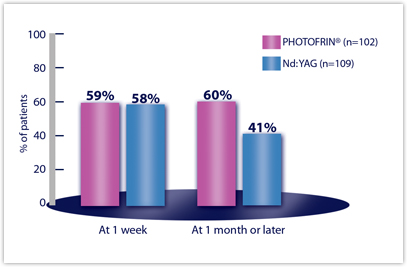
Objective tumor response rates (CR + PR), which demonstrate reduction of obstruction, were 59% for PDT and 58% for Nd:YAG at Week 1. The response rate at 1 month or later was 60% for PDT and 41% for Nd:YAG
Efficacy Results from Studies in Late-Stage Obstructing Endobronchial Cancer — All Randomized Patientsa
| EFFICACY PARAMETER | PDT N=102 | Nd:YAG N=109 |
|---|---|---|
| OBJECTIVE TUMOR RESPONSEb | ||
| Week 1 | 59% | 58% |
| Month 1 or later | 60% | 41% |
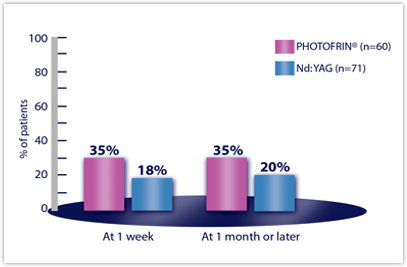
| ATELECASIS IMPROVEMENTc | n=60 | n=71 |
| Week 1 | 35% | 18% |
| Month 1 or later | 35% | 20% |
aStatistical comparisons were precluded by the amount of missing data at Month 1 or later (e.g., for tumor response, PDT 28% missing, Nd:YAG 38%).
bCR+PR where CR = complete response (absence of bronchoscopically visible tumor) and PR = partial response (increase ≥50% in the smallest luminal diameter; or any appearance of a lumen for completely obstructing tumors).
cIn patients with atelectasis at baseline.
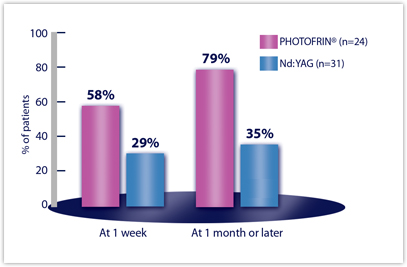
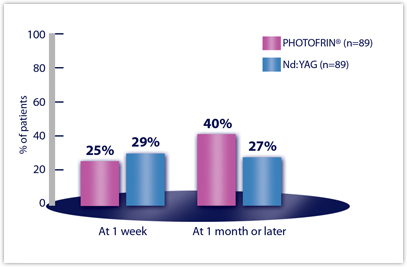
Efficacy Results from Studies in Late-Stage Obstructing Endobronchial Cancer — Clinically Significant Improvements in Patients with Moderate to Severe Symptoms at Baselinea
| CLINICALLY SIGNIFICANT SYMPTOM IMPROVEMENTb | PDT N=102 | Nd:YAG N=109 |
|---|---|---|
| ANY SYMPTOM | n=89 | n=89 |
| Week 1 | 25% | 29% |
| Month 1 or later | 40% | 27%a |
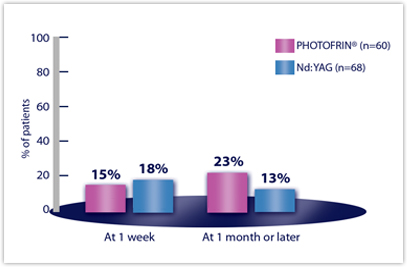
| DYSPNEA | n=60 | n=68 |
| Week 1 | 15% | 18% |
| Month 1 or later | 23% | 13% |
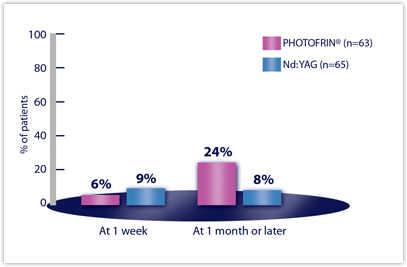
| COUGH | n=63 | n=65 |
| Week 1 | 6% | 9% |
| Month 1 or later | 24% | 8% |
| HEMOPTYSIS | n=24 | n=31 |
| Week 1 | 58% | 29% |
| Month 1 or later | 79% | 35% |
aStatistical comparisons were precluded by the amount of missing data at Month 1 or later.
bDyspnea was graded on a 6-point scale; cough and hemoptysis on a 5-point scale. Clinically significant improvement was defined as a change of at least two grades from baseline.
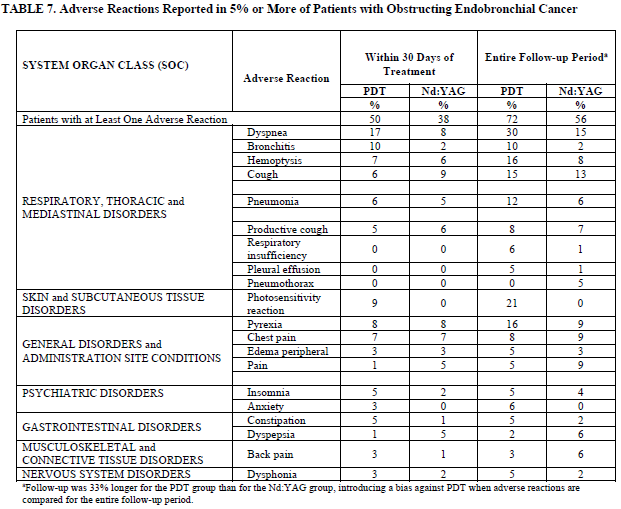
Adverse Reactions Reported in 5% or More of Patients with Obstructing Endobronchial Cancer
| SYSTEM ORGAN CLASS (SOC) | Adverse Reaction | Within 30 Days of Treatment | Entire Follow-up Perioda | ||
|---|---|---|---|---|---|
| PDT % | Nd:YAG % | PDT % | Nd:YAG % | ||
| Patients with at Least One Adverse Reaction | 50 | 38 | 72 | 56 | |
| RESPIRATORY, THORACIC and MEDIASTINAL DISORDERS | Dyspnea | 17 | 8 | 30 | 15 |
| Bronchitis | 10 | 2 | 10 | 2 | |
| Hemoptysis | 7 | 6 | 16 | 8 | |
| Cough | 6 | 9 | 15 | 13 | |
| Pneumonia | 6 | 5 | 12 | 6 | |
| Productive cough | 5 | 6 | 8 | 7 | |
| Respiratory insufficiency | 0 | 0 | 6 | 1 | |
| Pleural effusion | 0 | 0 | 5 | 1 | |
| Pneumothorax | 0 | 0 | 0 | 5 | |
| SKIN and SUBCUTANEOUS TISSUE DISORDERS | Photosensitivity reaction | 9 | 0 | 21 | 0 |
| GENERAL DISORDERS and ADMINISTRATION SITE CONDITIONS | Pyrexia | 8 | 8 | 16 | 9 |
| Chest pain | 7 | 7 | 8 | 9 | |
| Edema peripheral | 3 | 3 | 5 | 3 | |
| Pain | 1 | 5 | 5 | 9 | |
| PSYCHIATRIC DISORDERS | Insomnia | 5 | 2 | 5 | 4 |
| Anxiety | 3 | 0 | 6 | 0 | |
| GASTROINTESTINAL DISORDERS | Constipation | 5 | 1 | 5 | 2 |
| Dyspepsia | 1 | 5 | 2 | 6 | |
| MUSCULOSKELETAL and CONNECTIVE TISSUE DISORDERS | Back pain | 3 | 1 | 3 | 6 |
| NERVOUS SYSTEM DISORDERS | Dysphonia | 3 | 2 | 5 | 2 |
aFollow-up was 33% longer for the PDT group than for the Nd:YAG group, introducing a bias against PDT when adverse reactions are
compared for the entire follow-up period.
PHOTOFRIN® is indicated for the reduction of obstruction and palliation of symptoms in patients with completely or partially obstructing endobronchial NSCLC.
IMPORTANT SAFETY INFORMATION
Esophageal Cancer
PHOTOFRIN® is indicated for the palliation of patients with completely obstructing esophageal cancer, or of patients with partially obstructing esophageal cancer who, in the opinion of their healthcare provider, cannot be satisfactorily treated with Nd:YAG laser therapy.
Endobronchial Cancer
PHOTOFRIN is indicated for the treatment of microinvasive endobronchial non-small-cell lung cancer (NSCLC) in patients for whom surgery and radiotherapy are not indicated.
PHOTOFRIN is indicated for the reduction of obstruction and palliation of symptoms in patients with completely or partially obstructing endobronchial NSCLC.
High-Grade Dysplasia in Barrett’s Esophagus
PHOTOFRIN is indicated for the ablation of high-grade dysplasia in Barrett’s esophagus patients who do not undergo esophagectomy.
IMPORTANT WARNINGS AND PRECAUTIONS USING PHOTOFRIN® INCLUDE:
Gastroesophageal Fistula and Perforation: Do not initiate PHOTOFRIN with photodynamic therapy (PDT) in patients with esophageal tumors eroding into the trachea or bronchial tree or bronchial wall.
Pulmonary and Gastroesophageal Hemorrhage: Assess patients for tumors eroding into a pulmonary blood vessel and esophageal varices. Do not administer light directly to an area with esophageal varices.
High-Grade Dysplasia (HGD) in Barrett’s Esophagus (BE): After treatment of HGD in BE, conduct endoscopic biopsy surveillance every 3 months, until 4 consecutive negative evaluations for HGD have been recorded.
Photosensitivity and Ocular Photosensitivity: Observe precautions to avoid exposure of skin and eyes to direct sunlight or bright indoor light for at least 30 days. Instruct patients when outdoors to wear dark sunglasses which have an average light transmittance of <4% for at least 30 days and until ocular sensitivity resolves.
Use Before or After Radiotherapy: Allow 2-4 weeks between PDT and subsequent radiotherapy.
Chest Pain: Substernal chest pain can occur.
Airway Obstruction and Respiratory Distress: Administer with caution to patients with tumors in locations where treatment-induced inflammation can obstruct the main airway. Monitor patients closely between the laser light therapy and the mandatory debridement bronchoscopy for any evidence of respiratory distress.
Esophageal Strictures: Esophageal strictures can occur.
Hepatic and Renal Impairment: Patients with hepatic or renal impairment may need longer precautionary measures for photosensitivity.
Thromboembolism: Thromboembolic events can occur.
Embryo-Fetal Toxicity: May cause embryo-fetal toxicity. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception.
MOST COMMON ADVERSE REACTIONS reported during clinical trials (>10% of patients) are:
Esophageal Cancer: Anemia, pleural effusion, pyrexia, constipation, nausea, chest pain, pain, abdominal pain, dyspnea, photosensitivity reaction, pneumonia, vomiting, insomnia, back pain, pharyngitis.
Obstructing Endobronchial Cancer: Dyspnea, photosensitivity reaction, hemoptysis, pyrexia, cough, pneumonia.
Superficial Endobronchial Tumors: Exudate, photosensitivity reaction, bronchial obstruction, edema, bronchostenosis.
High-Grade Dysplasia in Barrett’s Esophagus: Photosensitivity reaction, esophageal stenosis, vomiting, chest pain, nausea, pyrexia, constipation, dysphagia, abdominal pain, pleural effusion, dehydration.
Other Photosensitizing Agents: May increase the risk of photosensitivity reaction.
Lactation: Because of the potential for serious adverse reactions in the breastfed infant, advise patients that breastfeeding is not recommended during treatment with PHOTOFRIN and for 5 months after the last dose.
Please see full Prescribing Information for PHOTOFRIN (porfimer sodium) for Injection at: www.photofrin.com.
FOR MORE INFORMATION about PHOTOFRIN, or if there are any questions regarding the information provided, visit www.photofrin.com or please contact the Medical Information Department at 1-866-248-2039. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
PHOTOFRIN® and OPTIGUIDE® are registered trademarks of Concordia Laboratories Inc.
Pinnacle Biologics™ and the logo of Pinnacle Biologics™ are trademarks of Pinnacle Biologics, Inc.
PHOTOFRIN® is distributed in the United States by Pinnacle Biologics, Inc., Bannockburn, IL 60015
All rights reserved.
Please see accompanying full Prescribing Information for Photofrin®
References:
- PHOTOFRIN® (porfimer sodium for injection) [package insert]. Bannockburn, IL: Pinnacle Biologics.
- Corti L, Toniolo L, Bosco C, et al. Long-term survival ofpatients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med. 2007; 39(5):394-402
- McCaughan JS Jr, Williams TE. Photodynamic therapy for endobronchial malignant disease: a prospective fourteen-year study. J Thorac Cardiovasc Surg. 1997; 114(6):940-946.
- Ross P Jr, Grecula J, Behaii-Saab T, Villalona-Calero M, Otterson G, Magro C. Incorporation of photodynamic therapy as an induction modality in non-small cell lung cancer. lasers Surg Med. 2006; 38(10): 881-889.
- Moghissi K, Dixon K, Stringer M, Freeman T, Thorpe A, Brown S. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases. Eur J Cardiothorac Surg. 1999; 15(1): 1-6.